Nicotinamide adenine dinucleotide, commonly known as NAD, is an essential molecule found in all living cells. It plays a vital role in metabolism and energy production and has been found to have numerous health benefits. Keep reading to learn more about what is NAD?
What is Nicotinamide Adenine Dinucleotide Responsible for in the Body?
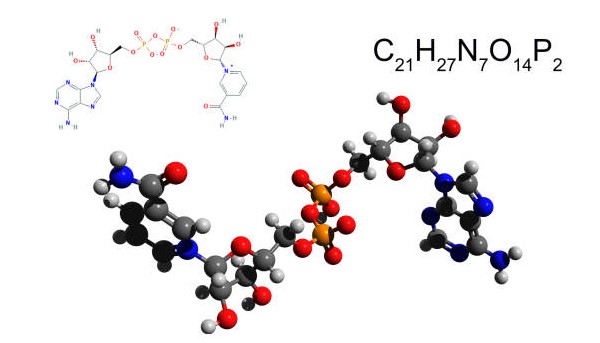
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. It’s composed of two molecules of adenosine monophosphate (AMP) and two molecules of nicotinamide. NAD plays an important role in numerous metabolic processes, such as energy production, DNA repair, and gene expression.
Nicotinamide adenine dinucleotide is essential for the production of energy, ATP. ATP, or adenosine triphosphate, acts as a source of energy for all life on Earth. It’s the energy currency of the cell and is responsible for powering nearly all of the biochemical reactions in the body. Without ATP, life as we know it would not exist.
NAD is also involved in the breakdown of carbohydrates, fats, and proteins. During this process, NAD helps to transfer electrons and hydrogen ions from one molecule to another. This helps to create the energy required for cellular activities. NAD can also be converted into NADP, a coenzyme needed for photosynthesis.
NAD is also involved in DNA repair. It helps to recognize and fix damaged DNA strands. This process helps maintain the genetic material’s integrity and prevents mutations. Furthermore, NAD is essential for the regulation of gene expression. It helps to activate or deactivate specific genes involved in various cellular activities.
In addition, NAD is involved in synthesizing certain hormones, such as insulin. Insulin is responsible for the regulation of blood sugar levels. NAD is also involved in the metabolism of drugs and toxins. This helps to detoxify the body and eliminate any harmful substances. Without NAD, these processes would not be possible, and the body would not be able to function correctly.
How does NAD become NAD+ and NADH?
The two primary forms of nicotinamide adenine dinucleotide (NAD) are NAD+ and NADH, which are interconvertible and play significant roles in the redox reactions of metabolism.
Redox reactions are essential for the efficient transfer of energy between molecules. In a redox reaction, electrons are transferred from one molecule to another, resulting in a change in the oxidation state of the molecules. NAD+ and NADH are involved in a wide variety of redox reactions, which are essential for efficient energy transfer between molecules.
NAD+ is the oxidized form of NAD, while NADH is the reduced form. NAD+ has a positive charge, while NADH has a negative charge. The transfer of electrons between these two forms is mediated by enzymes, which catalyze the reaction. The reaction involves the transfer of electrons from NAD+ to NADH, resulting in a decrease in the oxidation state of NAD+ and an increase in the oxidation state of NADH.
When NAD+ is reduced to NADH, energy is released as ATP. This energy can then be used for various metabolic processes, such as synthesizing proteins and fats. The reduction of NAD+ to NADH also maintains the balance of electrons in the cell, allowing for the efficient transfer of energy between molecules.
The conversion of NAD+ to NADH is an essential process in all living cells and is necessary for efficient energy transfer between molecules. Without this process, the cell would not function correctly, and the organism would be unable to survive.
Nicotinamide adenine dinucleotide plays an essential role in metabolic processes throughout the body. It’s used as a coenzyme for various redox reactions in the body and is involved in energy conversion, DNA repair, and gene regulation. NAD is also essential for the proper functioning of the immune system and the nervous system. Altogether, NAD is a vital molecule for cellular functioning and health.